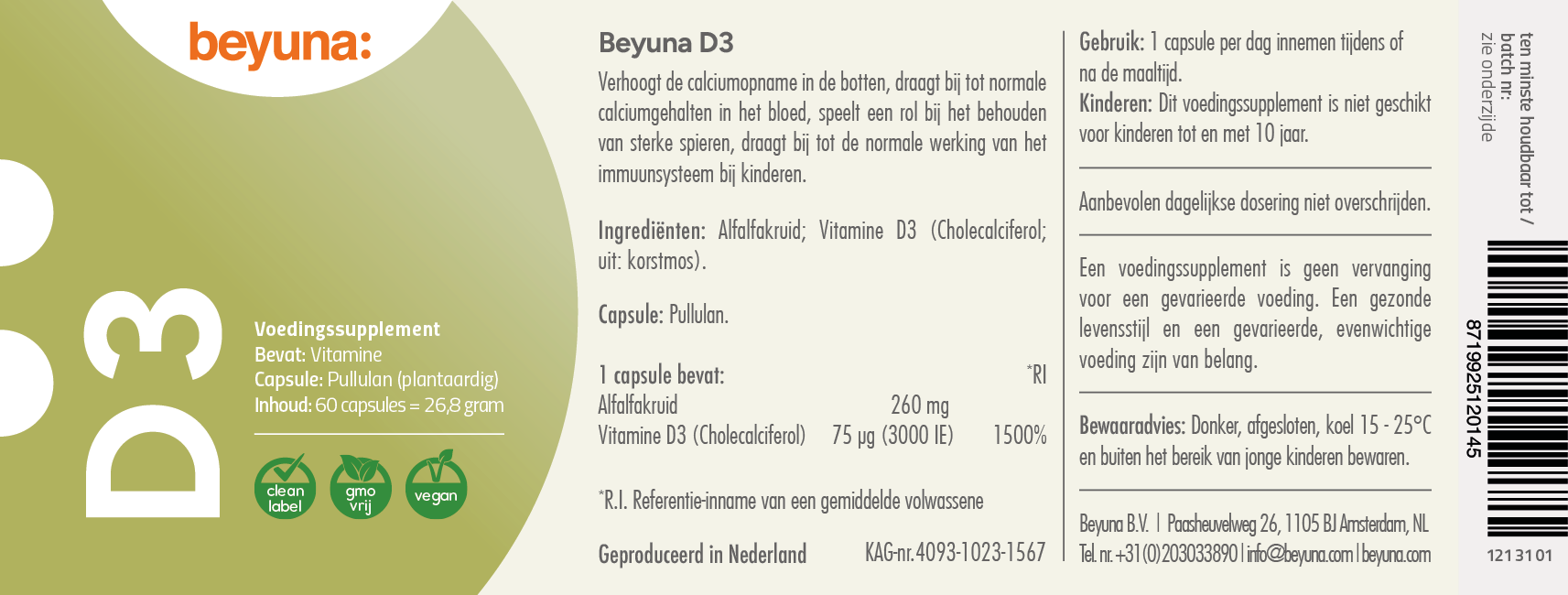
D3
A vegan D3 made from lichen and alfalfa herb. Lichen is a natural source of vitamin D3 (cholecalciferol). Beyuna D3 is Clean Label, meaning the supplement contains no synthetic fillers, additives, or colorants. The plant-based capsule is made from pullulan, which is naturally fermented from organic tapioca. Each capsule contains 75 µg (3000 IU) of vitamin D3.
BEYUNA D3
The recommended dosage for adults is 1 capsule per day.
Servings per container: 60
Amount per serving:
| Ingredients | % Daily Value | Amount | |
|---|---|---|---|
| Alfalfa herb | |||
| Vitamin D3 (cholecalciferol; from: lichen) | 1500% | (3000 IU) | 75.0 µg |
Capsule: vegetable (pullulan)
Vitamin D
- contributes to normal absorption/utilisation of calcium and phosphorus
- contributes to normal blood calcium levels
- contributes to the maintenance of normal bones
- contributes to the maintenance of normal muscle function
- contributes to the maintenance of normal teeth
- contributes to the normal function of the immune system
- has a role in the process of cell division
- The recommended daily dosage for adults is 1 capsule per day, taken with a glass of water during or after a meal.
- Do not exceed the recommended daily dosage.
- A dietary supplement is not a substitute for a varied diet. Maintaining good health requires a healthy lifestyle and a varied, balanced diet.
- Store in a dark, sealed place at a temperature of 15 - 25 °C, out of reach of young children.
BEYUNA D3
According to EC Directives 2000/13, 2003/89, 2005/26, 2005/63, 2006/142, 2007/68 and updates.
| Allergens | Yes/No |
|---|---|
| Cereals containing gluten and products thereof | No |
| Crustaceans and products thereof | No |
| Eggs and products thereof | No |
| Fish and products thereof | No |
| Peanuts and products thereof | No |
| Soy/ Soyderivatives | No |
| Milk and products thereof, including lactose | No |
| Nuts and products thereof | No |
| Celery and products thereof | No |
| Mustard and products thereof | No |
| Sesame seeds and products thereof | No |
| Sulphur dioxide and sulphites at concentrations of more than 10 mg/l, expressed as SO | No |
| Lupin and products thereof | No |
| Molluscs and products thereof | No |
GMO-Free
European legislation was adopted in 2012 relating to nutrition and health claims made on foods (the Commission Regulation). The Commission Regulation is a European Regulation (432/2012) which sets out what information may be provided regarding the effect of vitamins, minerals and other nutrients on products (on packaging, labels, websites, advertisements, in brochures, books and on social media).
Only approved health claims may be made. These claims provide information about the effect a particular ingredient has on health. Approved health claims are recommended by the European Food Safety Authority (EFSA). For many years, it was possible for companies to submit health claims, but unfortunately very few of them were approved. Because many of the claims submitted were rejected, some nutrients have no approved claims which relate to them.
Food supplements are not a medication and may only promote health. This means medical claims can never be made in relation to supplements.
We all enjoy free speech, and it is possible to make claims about the effect of certain nutrients in articles and brochures and on websites, but in this case there should be no advertising of a specific product or any reference made to it.
The Inspection Board for the Promotion of Health Products
Public advertising of self-care medicines, medical (self-care) aids or health products is in the interest of public health, though it does require clear regulations. Industry, the media and advertisers recognise their social responsibilities, and have created rules with which public advertising must comply.
These rules are drawn up by the Dutch Foundation for Monitoring Medicinal Product Advertising (KOAG) and the Dutch Foundation for the Promotion of Health Products (KAG). On behalf of the KOAG and KAG, the Inspection Board monitors the public advertising of medicines, medical (self-care) aids and health products.
Companies can have their claims verified by KOAG KAG. If a claim is verified and permitted, the company is given an approval number. This can be found on claims made in, e.g., brochures.
Product review
There are no reviews yet, log in and write the first!
Write a review about 'D3'
Please sign in to write a review.


.png)