Protein
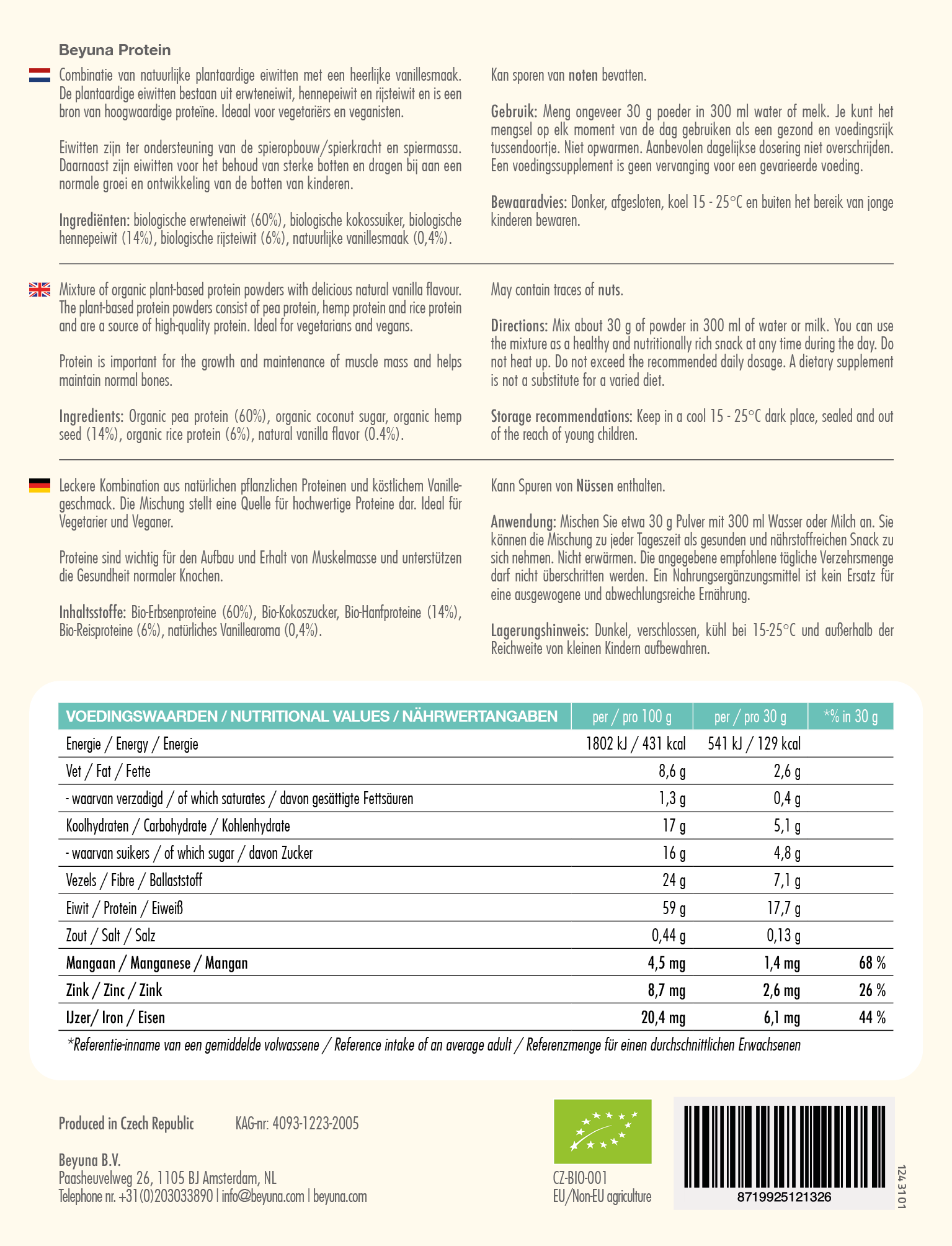
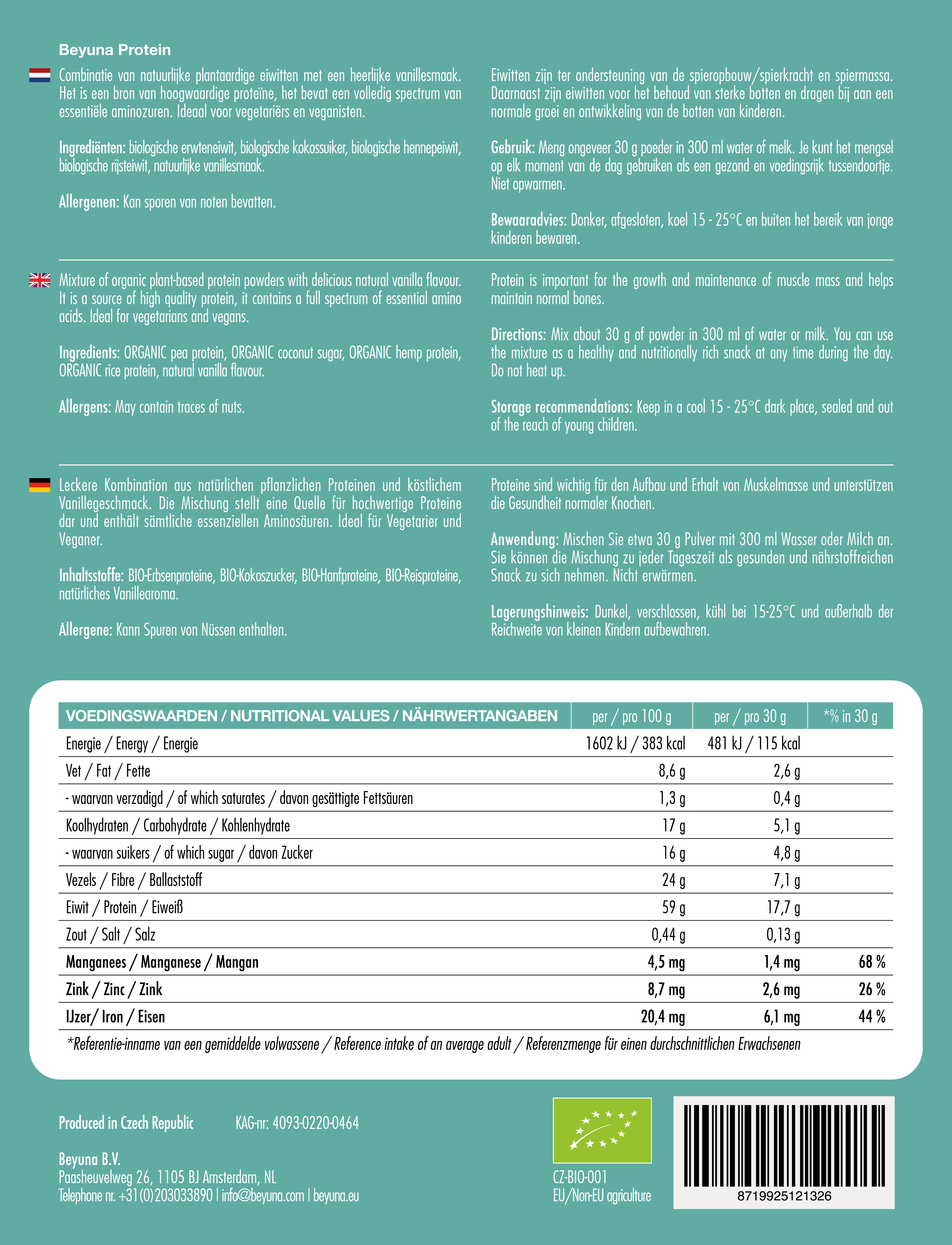
Combination of natural plant-based proteins from peas, hemp, and rice with a subtle vanilla flavor. This Protein contains a full spectrum of essential amino acids*. A source of high-quality protein, ideal for vegetarians and vegans!
Allergens: May contain traces of nuts.
- Label
- Ingredients
- Nutritional Values
- Amino acid profile*
- Benefits
- Usage
- Allergen list
- Commission Regulations
BEYUNA PROTEIN
| Ingrediënten |
|---|
| Organic pea protein |
| Organic coconut sugar |
| Organic hemp protein |
| Organic rice protein |
| Natural vanilla flavour |
BEYUNA PROTEIN
| Nutrutional Values | per 100 g | per 30 g | *R.I. in 30 g |
|---|---|---|---|
| Energy | 1602 kJ / 383 kcal | 481 kJ / 115 kcal | |
| Fat | 8,6 g | 2,6 g | |
| - of which saturates | 1,3 g | 0,4 g | |
| Carbohydrates | 17 g | 5,1 g | |
| - of which sugar | 16 g | 4,8 g | |
| Fibre | 24 g | 7,1 g | |
| Protein | 59 g | 17,7 g | |
| Salt | 0,44 | 0,13 g | |
| Manganese | 4,5 mg | 1,4 mg | 68% |
| Zinc | 8,7 mg | 2,6 mg | 26% |
| Iron | 20,4 mg | 6,1 mg | 44% |
*R.I. = Reference Intake of an average adult
BEYUNA PROTEIN
| Amino acid | Per 100 g |
|---|---|
| Alanine | 2,68 |
| Arginine | 5,27 |
| Aspartic Acid | 6,69 |
| Cystine | 0,63 |
| Glutamic acid | 10,22 |
| Glycine | 2,57 |
| Histidine | 1,61 |
| Isoleucine | 2,82 |
| Leucine | 4,94 |
| Lysine | 4,25 |
| Methionine | 0,68 |
| Phenylalanine | 3,2 |
| Proline | 2,17 |
| Serine | 3,41 |
| Threonine | 2,17 |
| Tryptophan | 0,55 |
| Tyrosine | 2,12 |
| Valine | 3,21 |
Protein
- supports rapid muscle recovery after training/sport performance
- contributes to the growth of muscle mass
- contributes to the maintenance of muscle mass
- supports muscle building/strength/mass
- for the maintenance of strong bones
- plays a role in bone formation
- is necessary for the normal growth and development of children's bones
- supports children's bones
- is important for the composition of children's bones
- Mix approximately 30 grams of protein in 300 ml of water or milk. You can use the mixture at any time of the day as a healthy and nutritious snack.
- A dietary supplement is not a substitute for a varied diet. For maintaining good health, a healthy lifestyle and a varied, balanced diet are important.
- Store in a dark, sealed place, at a temperature of 15 - 25 °C, and out of reach of young children.
BEYUNA PROTEIN
Allergen list according to EC Directives 2000/13, 2003/89, 2005/26, 2005/63, 2006/142, 2007/68 and updates.
| Allergens | Yes/No |
|---|---|
| Cereals containing gluten and products thereof | No |
| Crustaceans and products thereof | No |
| Eggs and products thereof | No |
| Fish and products thereof | No |
| Peanuts and products thereof | No |
| Soy/ Soyderivatives | No |
| Milk and products thereof, including lactose | No |
| Nuts and products thereof | Yes* |
| Celery and products thereof | No |
| Mustard and products thereof | No |
| Sesame seeds and products thereof | No |
| Sulphur dioxide and sulphites at concentrations of more than 10 mg/l, expressed as SO | No |
| Lupin and products thereof | No |
| Molluscs and products thereof | No |
*May contain traces of nuts.
GMO-Free
European legislation was adopted in 2012 relating to nutrition and health claims made on foods (the Commission Regulation). The Commission Regulation is a European Regulation (432/2012) which sets out what information may be provided regarding the effect of vitamins, minerals and other nutrients on products (on packaging, labels, websites, advertisements, in brochures, books and on social media).
Only approved health claims may be made. These claims provide information about the effect a particular ingredient has on health. Approved health claims are recommended by the European Food Safety Authority (EFSA). For many years, it was possible for companies to submit health claims, but unfortunately very few of them were approved. Because many of the claims submitted were rejected, some nutrients have no approved claims which relate to them.
Food supplements are not a medication and may only promote health. This means medical claims can never be made in relation to supplements.
We all enjoy free speech, and it is possible to make claims about the effect of certain nutrients in articles and brochures and on websites, but in this case there should be no advertising of a specific product or any reference made to it.
The Inspection Board for the Promotion of Health Products
Public advertising of self-care medicines, medical (self-care) aids or health products is in the interest of public health, though it does require clear regulations. Industry, the media and advertisers recognise their social responsibilities, and have created rules with which public advertising must comply.
These rules are drawn up by the Dutch Foundation for Monitoring Medicinal Product Advertising (KOAG) and the Dutch Foundation for the Promotion of Health Products (KAG). On behalf of the KOAG and KAG, the Inspection Board monitors the public advertising of medicines, medical (self-care) aids and health products.
Companies can have their claims verified by KOAG KAG. If a claim is verified and permitted, the company is given an approval number. This can be found on claims made in, e.g., brochures.
Product review
There are no reviews yet, log in and write the first!
Write a review about 'Protein'
Please sign in to write a review.